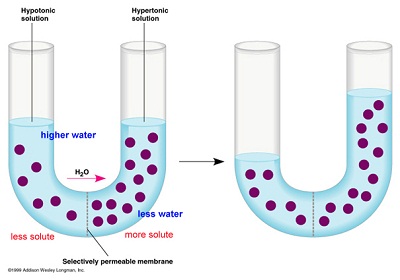
Hey Period 8+9, this was what Monday's lecture was about:
The are different types of cells. There are Prokaryote Cells and Eukaryote Cells which are 2 different domains. The Prokaryotic Bacteria Domain, the cells don't have Membrane Bound organelles and are not specialized. They old consist of Ribosomes and a Cell Membrane/Wall. With Eukarote Domain, there are 2 main cells, the Animal Cells (Of the Animal Kingdom) and the Plant cells (Of the Plant Kingdom). Both these cells have their differences.

Only Plants have a large Central Vacuole, Chloroplasts (for photosynthesis), Centrioles and a Cell Wall.
Now what makes Prokaryotes and Eukaryotes different you ask? Well the answer is organelles. Eukaryotic cells (yes, both plant and animal) have specialized structures withspecialized functions for example cillia or flagella for locomotion. Another reason is that they have "Containers." The cell has compartments in which different local environments are created for example separate pH's. They also have distinct and incompatible functions for example the lysosomes which has its own digestive enzymes. If the lysosome didnt have its own compartment the whole cell would be digested. Also membranes are the sites of Chemical Reactions. There are a unique combination of lipids and proteins and embedded enzymes and reaction centers for example the Choroplasts and Mitochondria where reactions occur.

Now how do cells "make their living"? What jobs do they have to do?
Well their first job is to build proteins. This is because PROTEINS CONTROL EVERYTHING! All the cell functions are controlled by proteins. Their second job is to make energy. This is in order to continue on in daily life and for growth to occur. The third and final job is for the creation of more cells. This gives the opportunity of growth, reproduction and most importantly repair.
Now it is important to study the production of proteins because they are important macromolecules. DNA (Deoxyribnucleic Acid) is the code for creating proteins. Proteins have the job of acting as an enzyme (most enzymes are proteins). Life cannot be run without the influence of proteins. Now in building proteins such organelles as the nucleus, ribosomes, the endoplasmic reticulu, the golgi apparatus and vesicles are involved. Now in the nulcues there obviously is DNA. Proteins go down an assembly line starting with the Nucleus (DNA),onward to the Ribosomes, then to the Endoplasmic Reticulum, the to the Golgi Apparatus and finally to the vesicles.

Now in creating proteins DNA cannot leave the cell, therfore it must make copies of itself inorder to leave and create the proteins that will later have specific functions. There is also no diffusion in the membranes because they are made of lipids. So the RNA travels through out the ER where the ribosomes dwell and read the code to create a polypeptide. Then they travel farther in the ER until they get to the end and bud off in a vesicle to the Golgi Apparatus. There the polypeptide finally folds istelf and travels farther until it is a completed and finsished protein on its way to do its job. An example of this would be the creation of Insulin and the Beta Cells of the Pancreas. If this producton stopped within 3 days a human would be dead.
The next important objective is to create energy in a cell. Once again making energy allows for daily life to continue and for growth to keep occuring. Now cells need lots of energy for power. In order to make energy, cells need to take in food and digest it, take in oxygen and therefore create ATP! (Adenosine triphosphate). Lastly the removal of waste is also needed. On to the Lysosomes!
Now the lysosomes are known as the "little stomach" of the cell (which is a misnomer because in humans digestion takes place mostly in the intestines). The lysosomes digest the macromolecules. The lysosomes are also the "clean up crew" of the cell because they break down the old and damaged organelles. Where Old Organelles go to die! Lysosomes are composed of vesicles with specialized digestive enzymes. Lysosomal Enzymes work best at a pH of about 4.8-5.0. The lysosome creates its own pH levels. It is more acidic than the rest of the cell. This is because the proetins in the lysosome membrane pump up H+ ions from the cytosol into the lysosome. The Cytoplasm happens to be all the contents of the cell while the cytosol is the "Gunk" between the organelles. Now, because the enzymes are sensitve to certain pH's they have to custom make their own and why is that? Well, enzymes are proteins aren't they? So what do we know about them? Yep, pH affects the protein structure and they can denature themselves. Now this is an adaption because if the digestive enzymes were to leak into the cytosol the cell would literally digest itself! But sometimes it is necessary for cells to die and be re-absorbed. Lysosomes can be used to kill cells when it is necessary. Sometimes proper development in an organism requires this process. Apoptosis, an "auto-destruct" process the lysosomes break open and kill the cell. For example the tail of a tadpole gets reabosorbed when it turns into a frog in order to grow legs. Or the loss of webbing between a fetuses fingers during its development (although there are some diseases such as Syndactyly in which the fingers are not dissolved and the fingers stay fused)

and the self-destruction of a cancerous cell in an organisms body (Obviously this doesnt always happen).

But as always, things do go wrong. The diseases of Lysosomes are most often fatal. This occurs when the digestive enzymes in the lysosomes fail to function correctly. What happens is that biomolecules are absorbed but not digested as they cant be. Therefore the lysosomes fill up with lots of undigested materials in which the cell grows larger and larger until the cell is disrupted along with the ogran functions. The are more than 40 known types of lysosomal storage diseases. For example Tay-Sachs disease in which the brain cells build up a number of undigested fats.

Well thats it for Monday's lecture. Tuesday's Sherpa will be Mark, Have Fun and Enjoy! :-) Night
-Liver